Supplement 7.3: Raman Scattering (2/6)
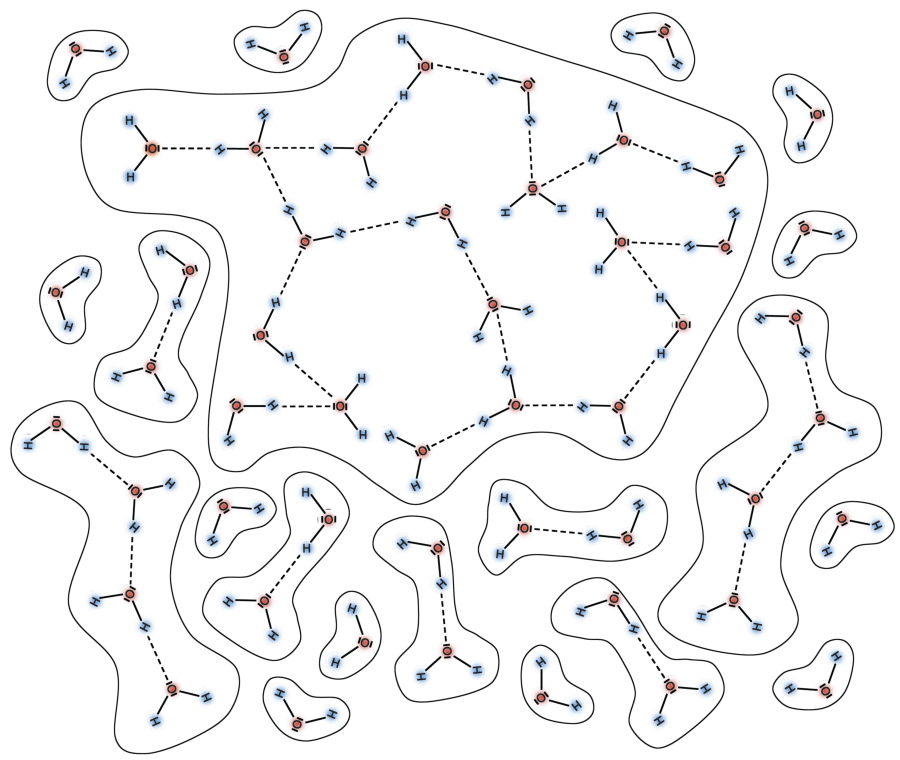
Hydrogen bonds
Molecules having a permanent dipole moment exhibit electric forces that attract other molecules close-by. In the water molecule, these are the hydrogen bonds. The diagram below shows how two water molecules are held together by a hydrogen bond.
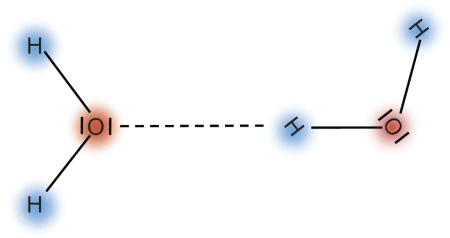
The bonding energy of the hydrogen bond is only about 1% of the bonding energy of the oxygen atom to either of the hydrogen atoms in the water molecule. For this reason, hydrogen bonds are not very stable: they break down and renew themselves repeatedly within a short period of time. Despite this, however, they play a crucial role in the properties of water: Hydrogen bonds are responsible for making water liquid at room temperature. A great number of molecules are held together by hydrogen bonds to form bigger aggregates of molecules just like polymers (also known as clusters). When liquid water evaporates, individual molecules leave the liquid state and enter the gas state. Water vapor is made up primarily of single water molecules, thus making water a monomer.
When temperature rises, the number and size of clusters decrease. Compared to single molecules, clusters take up a much bigger space owing to the length of the hydrogen bonds. On the other hand, when temperature rises, the thermal movement of molecules also increases, thereby necessitating much more space. This property is common to all matter and is known as thermal expansion. Both effects being present in liquid water lead to a density maximum (or a minimum of the specific volume) of water at 4°C.
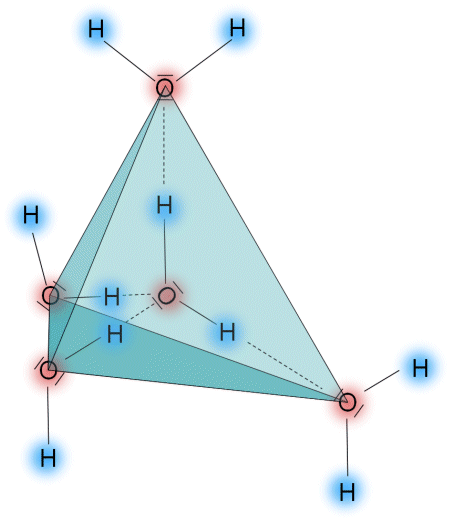
When water freezes into ice, all water molecules are held together by hydrogen bonds. Each molecule made up of an oxygen atom attached to two hydrogen atoms builds two additional hydrogen bonds that attract oxygen atoms of neighbouring molecules. The structure arising out of this process, as shown in the diagram below, occupies plenty of space causing water to expand by approximately 10% when freezing. Correspondingly, the density of frozen water is 10% less than the density of liquid water.
In comparison: Carbon dioxide is also triatomic, its chemical structure is O=C=O, but atoms are arranged not at an angle like water but rather symmetrically around an axis. Hence, it does not have a permanent dipole moment. For this reason, there are no permanent intermolecular dipole forces and carbon dioxide remains in a gas state at room temperature and atmospheric pressure.