Supplement 7.3: Raman Scattering (1/6)
Electric dipoles
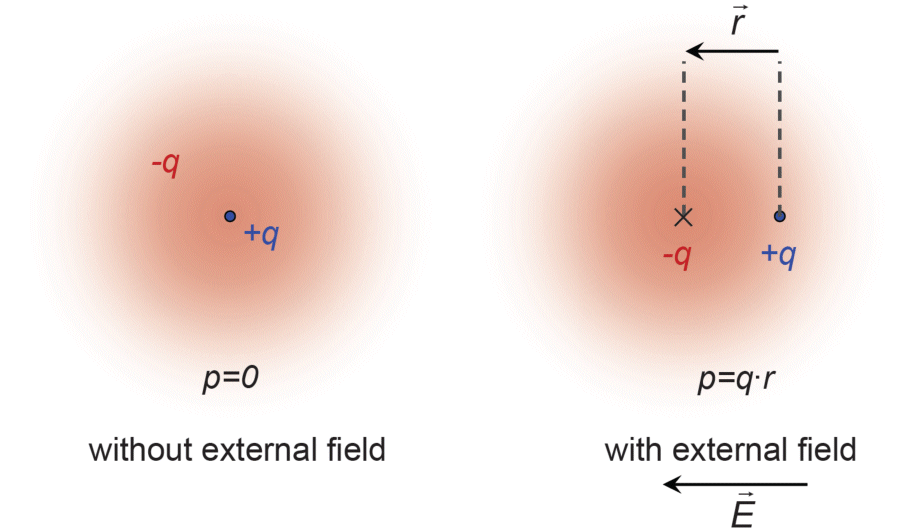
Let's take a look at an atom and its nucleus (charge q), as well as its electron shell (charge -q). The electrons move within the space around the nucleus, but in the average over time, the centroid of the negatively charged electrons and the positively charged nucleus coincide.
In an external electrical field a force
acts on the electrons. Owing to this force, the centroid of the electron shell moves with respect to the nucleus at a distance of . The distance between the centroids of the positive and negative charges leads to an electrical dipole moment
Electrical dipoles due to electrical fields are known as induced dipoles.
In addition to dipole moments that are caused by external electric fields, some molecules also have dipole moments that are permanent. These occur when the molecular structure is not symmetric around an axis or spherically symmetric.
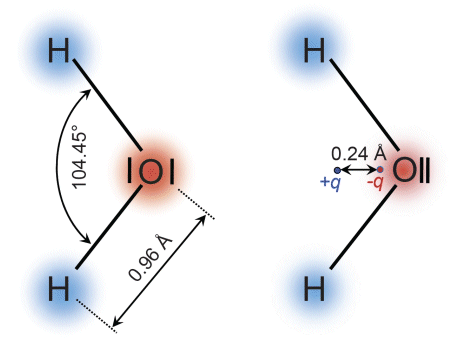
One example is the water molecule: the two hydrogen atoms are oriented at an angle of 104.45° to the oxygen atom. Since the bonding electrons are more likely located around the oxygen atom (oxygen having a higher electronegativity than hydrogen), the centroids of the positively charged nucleus and the negatively charged electrons do not coincide, but rather maintain the distance r=0.24 Å (1 Å=10-10 m) between them. This causes a dipole moment p=2·eo·r=7.7·10-30 C·m. Such molecules are known as permanent dipoles.