Supplement 7.3: Raman Scattering (3/6)
Molecular vibrations
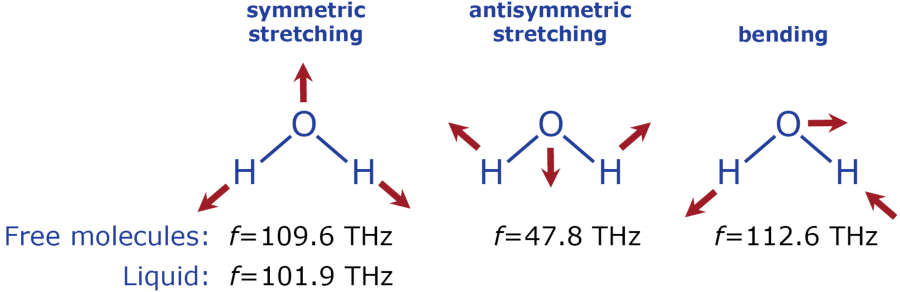
Molecules are made up of masses (the atoms) that are held together by bonding forces. These bonds are comparable to spring coils which enable the molecules to vibrate. Molecular vibrations are caused or changed by collisions. A molecule can vibrate in many different ways or modes, depending on the complexity of its structure. In a diatomic molecule, oxygen (O2)and nitrogen (N2) for instance, the two atoms vibrate against each other along a common molecular axis. The water molecule with its unsymmetrical structure can vibrate in three different modes, as shown in the diagram below. The frequencies of such vibrations can be computed out of the mass and bonding properties by using Hooke's Law. These are given in the following diagram.
Electromagnetic waves
An electric field produces electric dipoles in atoms and molecules with a dipole moment
In this equation, α is the polarizability, a substance-specific quantity that indicates how much an atom or a molecule can be polarised in an electrical field. Water molecules possess a permanent dipole moment that periodically changes owing to molecular vibrations. For its polarizability, we may write:
In this equation, αm refers to the average polarizability and αo refers to a polarizability which changes periodically due to the molecular vibration having the frequency fo.
Now let's take a look at an electromagnetic wave in water. Its electric field changes periodically according to:
where is the amplitude of the wave and f its frequency.
For the polarizability of the water molecule, we then derive:
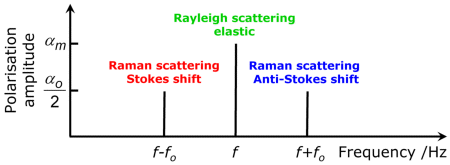
If we place the values of the polarizability resulting out of these equations and the values of the corresponding frequencies together, we obtain the following diagram:
Oscillating electrical charges emit electromagnetic waves. Therefore, the oscillating polarizability values of water molecule indicated here go hand in hand with the emission of such waves. They depict the scattered light that is produced when an electromagnetic wave strikes a water molecule.
- There is one component whose frequency is identical with the frequency f of the occuring wave. This is an elastic (which means energy-invariant) scattering known as Rayleigh scattering.
- Alongside this component are two others whose frequencies are shifted by the value of the vibrational frequency fo. This is an inelastic (not energy-invariant) scattering known as Raman scattering. The shift towards smaller frequency is called Stokes shift, whereas the shift to higher frequency is called anti-Stokes shift.
The result shows that a variable polarizability (αo>0) resulting from molecular vibrations is a condition for the occurence of Raman scattering.
Obviously, the polarizabilities of the two Raman lines are identical and therefore, the intensity of scattering of both lines should be the same, too. But actually, the anti-Stokes line is 1000 times weaker than the Stokes line under normal conditions. This effect is difficult to understand with the electromagnetic theory. It can be better explained by using quantum theory.