Supplement 7.2: Fluorescence (1/2)
What is fluorescence?
We consider electromagnetic radiation which illuminates matter and is partly absorbed. If radiation is re-emitted at the same or higher wavelengths within a short time lapse of about a nanosecond as a consequence of absorption, this is denoted as fluorescence. In case emission occurs after a longer time of microseconds, seconds or minutes, this is called phosphorescence. Fluorescence and phosphorescence can occur with gases, liquids and solids.
Here we use the photon model rather than the electromagnetic wave model of light because it is much more appropriate for explaining and understanding fluorescence: the photon energy (Planck‘s constant h, frequency f of the electromagnetic wave) can be directly related to the energy changes of electrons upon transitions between quantised electron states. The wave and photon models of light are described in more detail in the SEOS tutorial Understanding Spectra from the Earth.
Fluorescence of atoms
Absorption takes place when atoms are irradiated with photons having an energy which equals the difference of two discrete energy level .
The electron transferred from energy level to level returns to the level , whereby a photon with the same energy is emitted. There is no predominant direction of the emitted photon with respect to the direction of the absorbed photon. Frequency and wavelength of absorbed and emitted photons are identical, therefore this process is denoted as resonance fluorescence. Absorption and emission are discussed in chapter 2 of this tutorial in more detail.
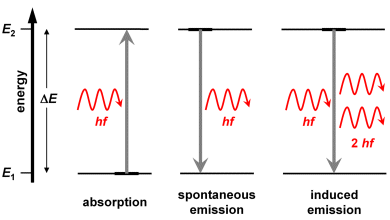
Atoms always have many electronic states at different energy levels. Therefore, an electronic transition from an excited state to the ground state can take place via intermediate states. The energy of emitted photons is then lower than the energy of the absorbed photon. Hence, this is not resonance fluorescence. The energy of fluorescence photons is lower (or the same, at best) than that of the absorbed photons, and the wavelength of fluorescent light is longer than that of the absorbed light.
Molecular states
Molecules are made up of groups of atoms bonded together by forces acting in between. These bonding forces are responsible for making molecules appear as individual units when viewed from outside. A molecule can be made up of a great number of atoms; macromolecules can have up to 104...106 atoms.
We consider a diatomic molecule as the simplest possible form of molecule, having a distance ro between the two atoms. Both attracting and repulsive forces which compensate at a separation ro must be present. Smaller distances lead to repulsion due to superposition of atomic electron orbitals (Coulomb repulsion) for which no common molecular orbitals exist. Larger distances yield predominantly attracting forces due to the bonding of common molecular orbitals (Coulomb attraction). The combination of both effects results in an energy relation with a minimum at the equilibrium separation ro.
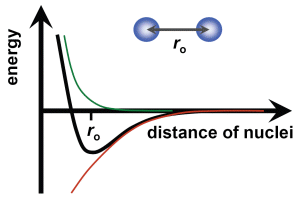
As with atoms, an outer electron can be excited by photon absorption to an electronic state at a higher energy level. The energy-versus-separation relation in the excited state is similar to the ground state. The equilibrium separation of the atoms is slightly larger since molecular bonding is weaker in the excited state.
The electron spin is an important parameter. In experiments, electrons are characterised by an angular momentum just like a sphere rotating around its centre (however, electrons are not spheres but point-like particles, and so this effect defies our perception). The electron spin is quantised, the spin quantum number s takes on two values only: ±½ (just like a sphere rotating clockwise or counter-clockwise, but again this picture does not hold with elementary particles). The spins of bonding electrons in the ground state are antiparallel (paired or opposite spins), and this is preserved when one electron is excited to a higher energy level. The state of two electrons having antiparallel spin is denoted as a singlet state.
It can happen - e.g., due to collisions with another atom or molecule - that the spin orientation of an excited electron undergoes a transition to a reversed orientation. Then the spin of the bonding electrons is parallel. The state of two electrons having parallel spins is denoted to be a triplet state. An excited electron in a triplet state cannot directly return to the ground (singlet) state, since its spin must revert to become antiparalllel again. This does not occur by itself but other atomic or molecular collisions are necessary due to the conservation law of angular momentum. Therefore, triplet states have a long lifetime.
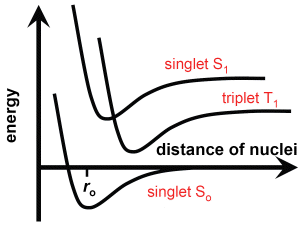
Transition of an electron from an excited to the ground state can result in a photon emission, just like atomic electron transitions. In a singlet transition, this takes place very quickly - the molecule fluoresces. In a triplet transition, this takes place more slowly - the molecule phosphoresces. Some molecules have a specific structure and transit immediately into a triplet state when excited; such molecules are denoted to be phosphorescent.
