2. Laser
By measuring their spectra, we were able to determine the fundamental difference between laser light and the light of a gas discharge. Now, how do we explain the process of producing laser light out of the light of the discharge? To understand this, we should first learn about the interaction between light and matter.
Absorption and emission
For the following deliberations, we shall be regarding light as particles, photons, see the SEOS tutorial Understanding Spectra from the Earth for more details. Photons propagate with the speed of light c. In vacuum (and approximately in air) it is c=2.998·108 m/s.
The photon energy is
where h=6.626·10-34 J s is Planck’s constant, and f is the frequency of the same light when considered as electromagnetic wave. With the relation
the photon energy can also be written with the wavelength λ of the electromagnetic wave:
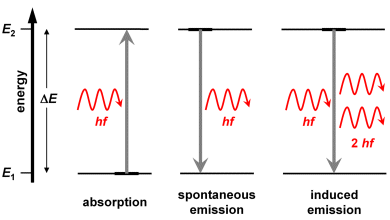
An interaction of photons with atoms or molecules can occur in three different ways (see also the graph in the right column):
-
Absorption
A photon strikes an atom and is immediately absorbed. The photon's energy is transferred to the atom, thus moving it into an excited state.
-
Spontaneous emission
An excited atom emits a photon and moves into a state of low energy (the lowest state is the ground state). The energy difference between the two states determines the wavelength of the emitted photon: . The direction of the emission is merely coincidental.
-
Induced emission
Pre-requisite for an induced emission by an atom or molecule in an excited state is the presence of a photon having the energy . When this photon interacts with the excited state, this results to the emission of another photon having the energy . This second photon is identical with the primary ‘inducing’ photon: it has the same energy and propagates into the same direction. In the wave model, both should exhibit identical wavelengths, phases and directions of propagation of the partial waves.
As mentioned in the left column, induced photons cannot be distinguished from the inducing photons. As a consequence, it is not only the photon energy but also the direction of propagation which are identical. This explains that laser light is well collimated (with most lasers, not all of them), illuminating a small spot also at a distance. The opening angle of a laser beam is denotes as divercence angle; laser beams have a low divergency. Read more about divergence angles in supplement 2 of chapter 3.
The applet below illustrates how photons and atoms can interact with one another. Please move the scroll bar seen in the applet to the right.
- Photons striking at a slow rate are absorbed by the atoms and emitted spontaneously (excited atoms are shown with a green ring).
- A higher photon rate causes an induced emission whenever a second photon strikes an already excited atom.
Principally, this is how laser light is obtained: induced emission occurs more frequently than absorption and spontaneous emission. Then light amplification exceeds the losses. The number of identical photons increase, thereby producing laser light.
Induced emission is therefore necessary in order to produce laser light.
However, before we can successfully produce laser light, we need to consider the balance of electron transitions in atoms...
