Supplement 7.2: Fluorescence (2/2)
Molecular states (cont.)
Besides electronic excitation by photons - discussed on the preceeding page - there are two other mechanisms of energy excitation with molecules:
- Vibrational excitation. Molecules oscillate due to the masses of atoms and the bonding forces in between. Vibration energies are not continuous but discrete ('quantised'). They fill up the energy minimum around the equilibrium separation ro with further energy levels. The energy differences of these levels (0.1 eV) are about 10 to 100 times less than the energies of atomic and molecular electronic excitation (1...10 eV). Despite this, the vibrational states are quite important for the characteristics of molecular fluorescence, as shown below.
- Rotational excitation. Molecules rotate about their mass centre. The rotation energies (0.01 eV) are approx. 10 times less than the vibrational energies.
Fluorescence of molecules
In gas molecules, all kinds of energy transfer mentioned above contribute to the complex fluorescence spectra of gases. Fluorescence spectra of liquids are much simpler (oils for example, see page 2 of chapter 7, or fluorescent molecules dissolved in liquids or bound to particles (for example yellow substance in water or chlorophyll a in phytoplankton, see page 3 of chapter 7).
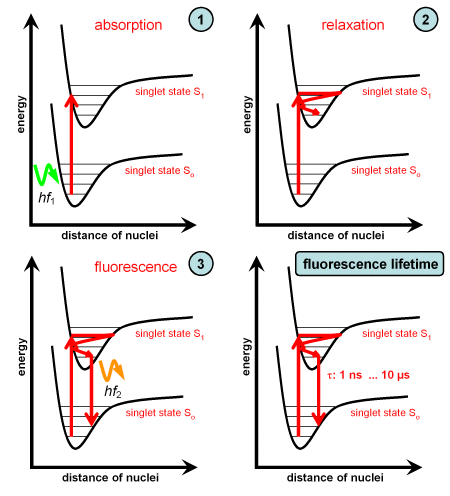
The graph in the left column depicts the processes which occur during fluorescence:
- A photon having an energy is absorbed, the molecule is transferred to an excited vibrational level of the singlet state S1.
- Starting at the excited vibrational level the molecule relaxes to the lowest vibrational level. The energy difference is released as heat to the environment.
- From the lowest vibrational level of the singlet state S1 the molecule transits to a vibrational level of the ground state S0. The energy difference is emitted as a fluorescence photon . Due to relaxaton it is . Since transition always starts at the lowest excited vibrational level, the energy of the fluorescence photon (and so the shape of the fluorescence emission spectrum) is invariant and independent of the absorbed photon energy.
Since both electron states are singlet states and the spin is unchanged, the molecule transits very rapidly to the ground state, hence the fluorescence lifetime is short.
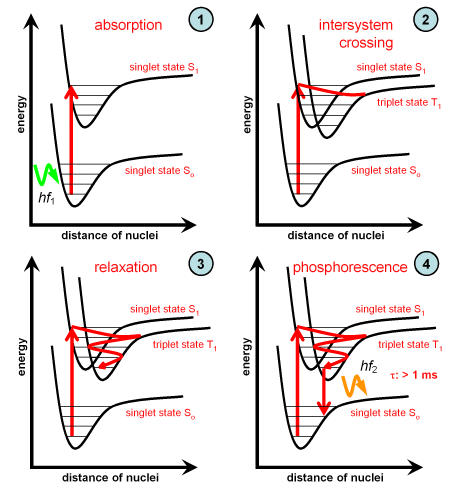
When the excited electron transits from a singlet to a triplet state this is denoted as intersystem crossing. The electron remains for a longer time in the triplet state before a transition to the ground state takes place, as shown in the graph below. Phosphorescence induced by this process has therefore a longer lifetime.