2. Laser
The Boltzmann distribution
A prior condition for producing laser light is is the induced emission of photons. This must occur more frequently than absorption and spontaneous emission. These balances are investigated more thoroughly in supplement 1. Another look at the graph on the previous page ...
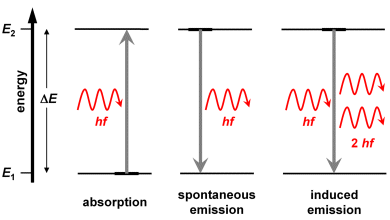
... shows that there must be more atoms in the higher energy state E2 than in the lower state E1: then photons having a suitable energy ΔE will provoke an induced emission instead of absorption. It is denoted as population inversion if the state E2 is occupied by more atoms than the state E1.
The term population inversion indicates that deeper energy levels are normally occupied by more atoms than the higher states. Denoting the number of atoms in state E1 as N1, and the number of atoms in state E2 as N2, then it is normally N1>N2. In case of a population inversion it should be: N1<N2.
Can we calculate N1 and N2? Possibly yes, if the system (the atoms or molecules) does not transmit or receive energy from outside. After a while, the values of N1 and N2 do not change any more and reach an equilibrium. This is called the thermodynamic equilibrium. The ratio of N1 and N2 is then given by the Boltzmann distribution
where T is the absolute (Kelvin) temperature. Obviously, the population ratio depends only on temperature! The factor k is the Boltzmann constant, it is k=1.38·10-23 J K.
The graph below shows a Boltzmann distribution. It has been calculated with energy levels that are used to produce the laser light of a HeNe laser having the wavelength 633 nm. Here it is: E2-E1=3.1·10-19 J.
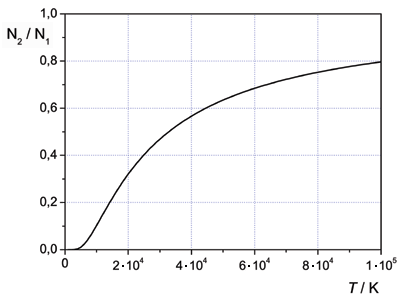
We observe that even with a temperature of 100 000 K, the level E1 has a higher population than E2. At room temperature, the population ratio is so small that it is quite indistinguishable from the zero line.
Lasers are apparently far from a thermodynamic equilibrium!
