Supplement 7.3: Raman Scattering (6/6)
Raman scattering of water (cont.)
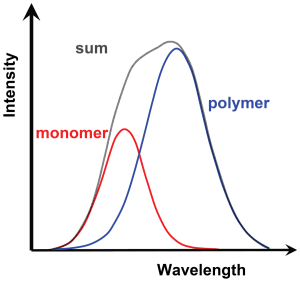
A closer look at the spectra of Raman scattering shown on the previous page reveals a certain structure in the Raman bands. The diagram below shows this in a higher spectral resolution. The structure is the result of the permanent dipole moment of the water molecule: as explained in previous pages, liquid water is made up not only of free individual molecules but also of molecules that are bound together as clusters by hydrogen bonds. The hydrogen bonds weaken the bonding energy of the oxygen atom to either of the two hydrogen atoms in a molecule, thereby causing the wave number to decrease and the wavelength of the Raman shift to increase.
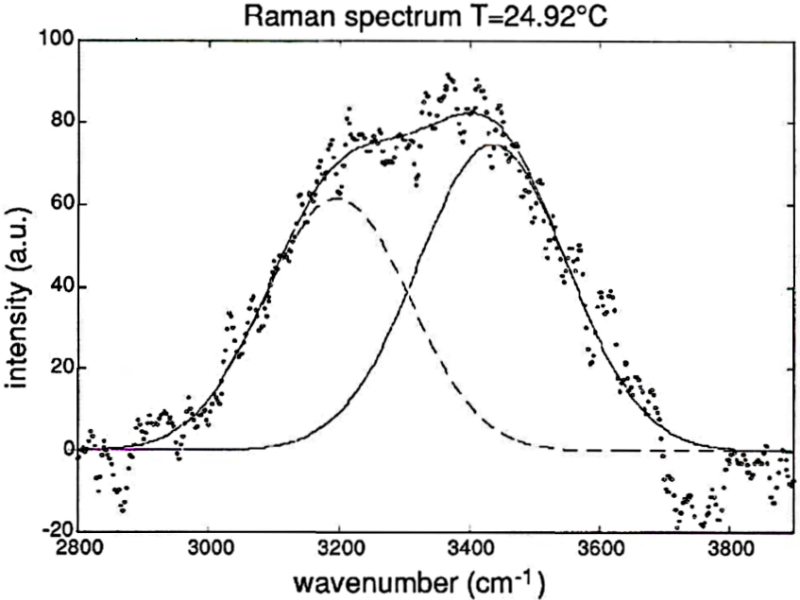
The relationship of monomer and polymer water molecules depends on temperature (see page 2). The sum spectrum of the two interfering Raman bands is likewise dependent on temperature, as can be observed in the diagram of experimental data shown below.
Source: EARSeL Advances in Remote Sensing
This brought up the idea to determine the temperature of liquid water out of the spectral signature of the Raman scattering. Technically, this method is much more complicated and difficult than measuring temperature with conventional thermometers. But it has one great advantage and that is, the possibility of determining water temperature from a great distance without physical contact - through remote sensing.