3. Climate and climate change
Solar Radiation (3/4)
The Fraunhofer lines (Cont.)
In reality, there are a many more absorption lines in the spectrum of solar radiation, since all the elements in the photosphere and chromosphere of the sun contribute to the aforementioned scattering of radiation. The following figure shows an example of the measurable lines in the range from 500 nm (blue-green) to 600 nm (orange).
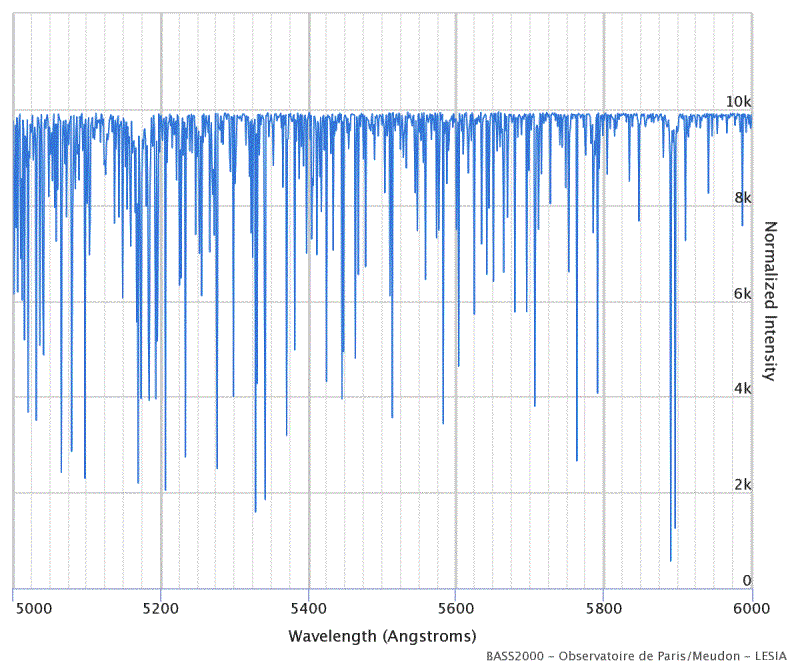
All Fraunhofer lines in the range from 5000 to 6000 Ångström (or 500 to 600 nm). The thermal spectrum of solar radiation has been removed, so that only Fraunhofer lines can be seen in the picture.
Source: BASS2000 Solar Survey Archive
The database BASS2000 from which the illustration is taken, can also display other spectral ranges. The lines interactively denote the elements responsible for the scattering, as well as most of the transitions between energy levels in the electron shells.
Compare the two images on the previous page with the illustration above. Can you find, in the BASS2000 spectrum, the two orange-colored lines labeled D, or D1+D2 at about 589 nm? They are created from sodium atoms and referred to in atomic physics as sodium doublet lines.
An experiment in physics class
The absorption by sodium atoms, as they occur in the solar atmosphere, can also be shown in a physics class. Among the equipment you can see a sodium fluorescent tube containing small amounts of metallic sodium. The oven, which is fitted with windows, is heated up to about 250°C, which causes the sodium to vaporise.
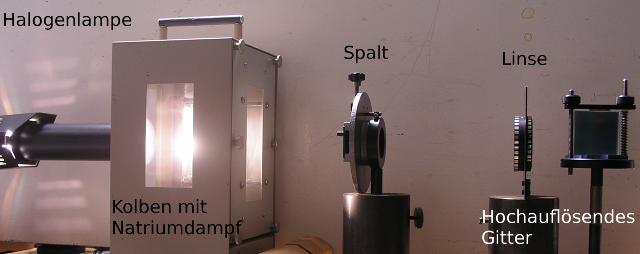
Experimental setup for the detection of the Fraunhofer lines of sodium.
Source: Vorlesungsexperimente am Institut für Physik, Universität Oldenburg
The sodium vapor in the tube is illuminated with a halogen lamp. The penetrating light is split up by diffraction with an optical grating and mapped onto a screen with a lens. The sodium doublet lines can be easily distinguished.
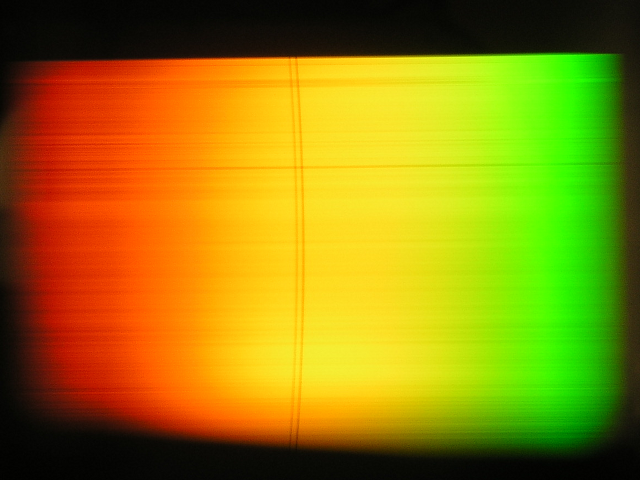
Spectrum of a halogen lamp with the Fraunhofer lines of sodium.
Source: Vorlesungsexperimente am Institut für Physik, Universität Oldenburg

