1. Light and Radiation
Photons (3/4)
What are the characteristics of photons?
-
They have a much, much higher velocity than particles which we encounter in our daily life.
In vacuum, they travel with the velocity
c=2.998·108 m/sThis is the speed of light, which was tackled under electromagnetic waves. Just like electromagnetic waves, photons have a slightly lower velocity when propagating through transparent matter. Photons can neither move faster nor slower than with the speed of light! - Photons do not have mass compared to other known particles in our daily life.
-
Photons possess energy E and momentum p:
The quantities f and λ correspond to the frequency and wavelength of electromagnetic waves.
- At first, these relations seem to be contradictory. They combine characteristics of particles (energy, momentum) and the characteristics of waves (frequency, wavelength), which in our understanding are apparently not related at all. However, this is very typical in quantum physics. Light possesses the characteristics of both waves and particles at the same time! This is known as wave-particle-dualism.
-
Waves and particles are complementary models of light:
-
Wave and particle characteristics are connected together through Planck’s constant
h=6.626·10-34 J s .Planck’s constant is one of the fundamental constants in quantum physics.
Mass, momentum and energy of particles and photons are explained more closely in supplement 1.5.
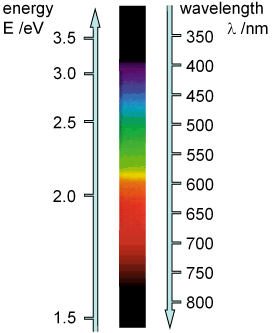
Which units are used to indicate photon energy? The unit Joule (or watt second) would result to very small numerical values of about 10-20 J for photons in the visible range. Therefore, the unit electronvolt (eV) is more preferred. It is not a SI unit. 1 eV is equivalent to 1.6·10-19 J in SI units.
Now we can try to give answers to the questions which we could not answer before: What is energy and what is intensity of light? Which type of light tans our skin? How do we see colour?...
Photons can be absorbed by atoms and molecules, provided that they possess the right amount of photon energy necessary for an absorption. Otherwise, an illuminated object is either transparent or reflective. Skin tanning is the result of a chemical reaction which requires photon energies of around 3 eV, which corresponds to that of blue or ultraviolet light having 400 nm wavelength. Therefore, visible light not having sufficient intensity in that wavelength range - such as that of a light bulb - is not capable to cause skin tanning.