What's the matter with ozone ?
Two different types of ozone
Ozone is a gas that occurs both in the Earth's upper atmosphere and at ground level.
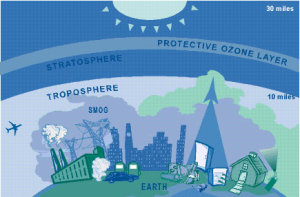
The layer closest to the Earth's surface is the troposphere. The troposphere generally extends to between 10 and 18 km up, where it meets the second layer, the stratosphere. Here, ground-level or "bad" ozone is an air pollutant which is harmful to breathe and damages crops, trees and other vegetation. It is a main ingredient of urban smog which can cause officials to issue "ozone alerts".
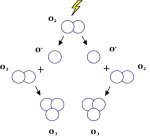
The stratosphere or "good" ozone layer protects life on Earth from the sun's harmful ultraviolet (UV) rays. Ozone is produced naturally in the stratosphere. "High-energy" UV radiation strikes O2 molecules which split into 2 free oxygen atoms. The free oxygen atoms collide with O2 molecules to form ozone molecules.
Ozone depletion
But this ozone layer is gradually being destroyed by chemical reactions which mainly involve light, chlorine and bromine. The main source of these atoms in the stratosphere is photodissociation of chlorofluorocarbon (CFC) and bromofluorocarbon compounds. These compounds, which are also active as greenhouse gases, are a result of human activity.
Thinning of the protective ozone layer can be observed by satellites, particularly over the polar regions, and is known as the ozone hole. Ozone depletion causes increased amounts of UV radiation to reach the Earth which can lead to more cases of skin cancer, cataracts and impaired immune systems.

The ozone hole is seasonal
The role of sunlight in ozone depletion is the reason why the Antarctic ozone depletion is greatest during spring and reaches its maximum area in September. In the polar regions (particularly at the south pole), circulation patterns generate little mixing in winter, whereas normally gases are well-mixed. This creates an atmospheric container which fosters the ozone-destroying chemical reactions.
By October, these reactions stop and the hole shrinks in area and depth. During the period from October to December, the ozone depleted region is "stirred up like a can of paint" into the mid-latitudes, depleting atmosphere ozone there.
Despite successful measures that have stopped production of CFCs, scientists don't expect to see the hole significantly reduce in size for about another decade. This is due to the long lifetimes of CFCs already in the atmosphere. Also scientists remain uncertain about how climate change will interact with the recovery of the ozone hole.
Ozone affects climate...
The more ozone in a given parcel of air, the more heat it retains. Ozone generates heat in the stratosphere, both by absorbing the sun's ultraviolet radiation and by absorbing upwelling infrared radiation from the troposphere. Consequently, decreased ozone in the stratosphere results in lower temperatures.
At the same time greenhouse gases - among which ozone - have risen in the troposphere, triggering global warming. The two phenomena may be linked.
...and climate affects ozone
You have to know that temperature, humidity, winds, and the presence of other chemicals in the atmosphere influence ozone formation, and the presence of ozone, in turn, affects those atmospheric constituents.
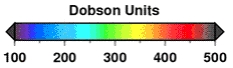
The ozone unit
The ozone vertical column density is expressed in Dobson units (DU). The Dobson unit is the most basic measure used in ozone research.
One Dobson unit refers to a layer of ozone that would be 10 µm thick under standard temperature and pressure. For example, 300 DU of ozone brought down to the surface of the Earth at 0°C would occupy a layer only 3 mm thick.
