Supplement 2.3: Emulsies
Waarom mengen olie en water niet?
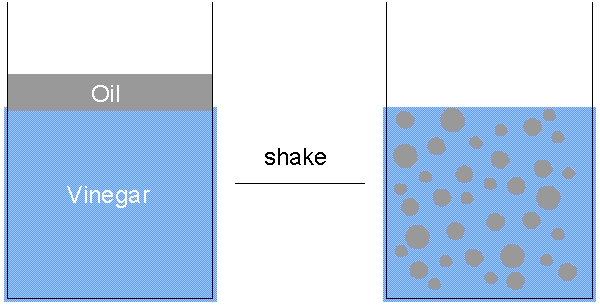
Sommige emulsies zijn vrij stabiel en zullen pas na lange tijd uit elkaar vallen. Melk is bijvoorbeeld een emulsie van water en vet, maar is vrij stabiel. Andere emulsies vallen vrij snel uit elkaar, bijvoorbeeld een eenvoudige saladedressing van olie en azijn zal vrijwel onmiddellijk uit elkaar vallen. Dit komt doordat olie minder dicht is dan azijn, dat op waterbasis is.
De emulsie zelf bestaat uit kleine druppeltjes van een vloeistof in een tweede vloeistof. Een emulsie die druppeltjes olie
in water bevat, wordt een olie-in-water-emulsie genoemd. De olie wordt dan de gedispergeerde fase genoemd,
terwijl het water de continue fase wordt genoemd.
n het andere geval, d.w.z. wanneer water de gedispergeerde fase is en olie de continue fase, is er sprake van een
water-in-olie-emulsie.
Dit proces wordt ook wel omgekeerde emulsificatie genoemd.
De fysica van emulgering
Alle oppervlakken hebben een oppervlakte-energie die verantwoordelijk is voor verschijnselen zoals oppervlaktespanning. Als je een druppel olie in een glas water doet, ontstaat er een nieuw oppervlak op het grensvlak tussen de olie en het water, en dit oppervlak heeft een energie.
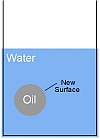
Deze energie moet ergens vandaan komen. Om een emulsie van olie en water te maken, moet je dus energie toevoegen! Je moet bijvoorbeeld een saladedressing schudden om een emulsie van olie en azijn te maken. Als je niet schudt, blijven de twee vloeistoffen in aparte lagen.
Alle systemen proberen hun energie tot een minimum te beperken. Zo wil het systeem van een druppel olie in water het oppervlak van het grensvlak tussen olie en water verkleinen. Een kleiner oppervlak betekent minder oppervlakte-energie. Voor een gegeven volume olie wordt het kleinst mogelijke oppervlak verkregen door een bol te vormen; daarom zijn oliedruppels in water en gasbellen in een vloeistof altijd bolvormig.
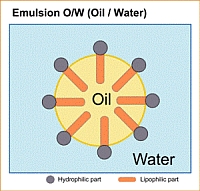
Olie-in-water-emulsies (o/w)
Bij o/w-emulsies is de waterfase continu, terwijl de oliefase in de vorm van kleine druppeltjes in het water is verdeeld. Een o/w-emulsie bevat ten minste 26% water.
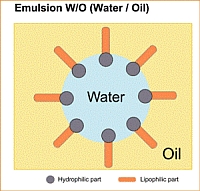
Water-in-olie-emulsie (w/o)
Bij w/o-emulsies zijn de omstandigheden omgekeerd: de oliefase is continu en het water vormt druppeltjes in de olie. Het watergehalte in w/o-emulsies bedraagt maximaal 74%.
Het type emulsie wordt echter niet bepaald door het watergehalte, maar door het type emulgator!
Emulgatoren
Olie-in-water-emulsies ontstaan wanneer emulgatoren met hydrofiele (waterminnende) groepen overheersen, terwijl hydrofobe (olieliefhebbende) groepen leiden tot de vorming van water-in-olie-emulsies. Een emulgator positioneert zich op het grensvlak tussen olie en water of lucht en water en heeft door de oppervlaktespanning te verminderen een stabiliserend effect op de emulsie.
Vraag:: Kun je voorbeelden bedenken van olie-in-water- en water-in-olie-emulsies uit je dagelijks leven?
