4. الامتصاص والتشتت
Some mathematics
We are starting with a focus on the smallest – infinitesimal – scale within an absorbing substance that is illuminated from the left hand side. The light has an intensity of at the point . Only an infinitesimal step further, at the point , the intensity has decreased by an infinitesimal amount , so it can be described as .
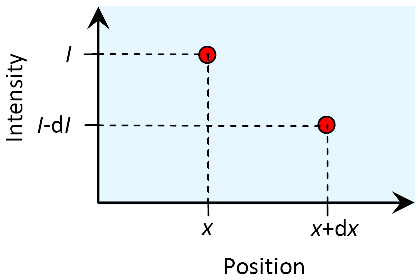
The decline is assumed to be proportional to and :
On top of that, the decline will be dependant on the absorption features of the substance. These special properties will be characterised by the absorption coefficient a, what makes proportionality become an equation:
Through transformation, the following differential equation is obtained:
which now has to be solved (or: integrated). When said in words, the solution is easy to find:
"Questioned is the intensity
, ..."
on the left: "...of which the differentiation with respect to
..."
on the right: "... results in
with the additional factor
."
The function that is able to do that is the exponential function: It persists when being derivated or integrated. A possible approach for solving the problem is thus:
what leads to the initial differential equation when being differentiated with respect to :
Therefore the exponential equation is the way to the solution.
At the point the intensity shall equal (Let this be the intensity at the beginning, the previous approach yields for the value of 1), and has to be completed on the right hand side of the equation. This leads to the final solution:
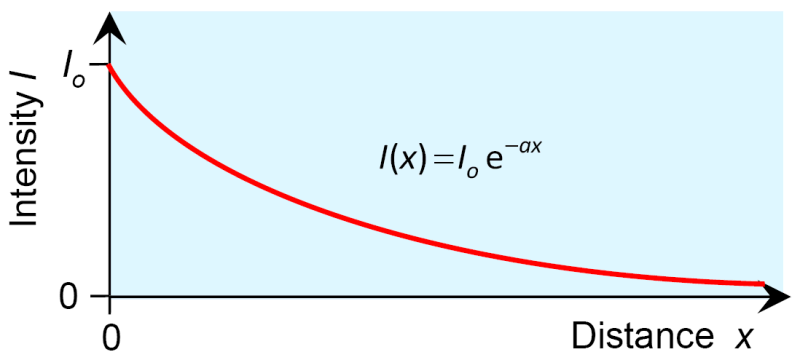
This is Lambert's law: The intensity of light decreases exponentially on its way through an absorbing medium, while the decline depends on the medium's absorption coefficient .
Source of the Chlorophyll a absorption spectrum: PhotochemCAD. The spectrum shows the molar decadic absorption coefficient, which will be explained in Supplement 4.1.
The dimension of the absorption coefficient is an inverse length. Depending on its value, it may be given in different units:
- for the cloud-free atmosphere mostly in 1/km,
- for waters in 1/m,
- for highly absorbing materials (e.g. spots of environmental pollution through crude oil) in 1/μm.

