2. Thermal radiation
Light as a particle: Planck's radiation law (2/2)
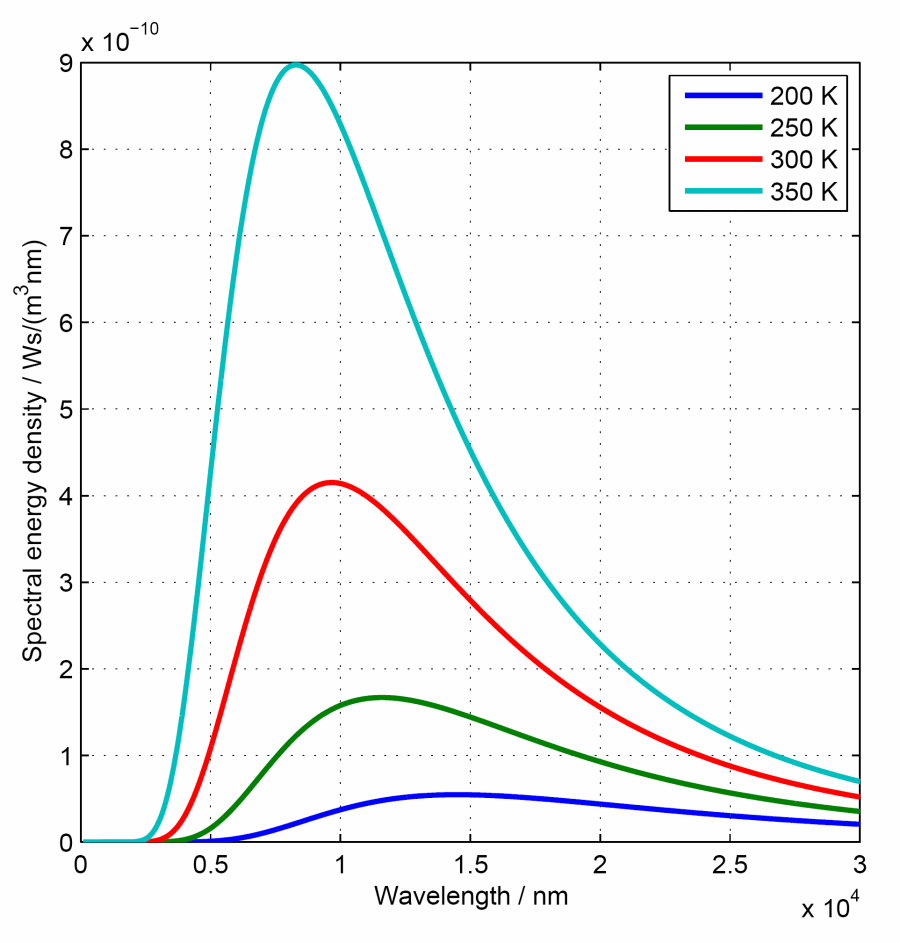
Radiation as a function of the wavelength
Microwaves are commonly indicated by their frequency whereas visible light is mostly characterised by the use of wavelengths. Planck's radiation law in dependency of the wavelengths indicates:
How to realise a cavity radiator, which emits radiation as a black body obeying Planck's radiation law? This significantly depends on the emitters temperature, in other words on the desired wavelength in the spectrum.
Planck emitters
At room temperature, an emitter which has a maximum radiation rate of about 10μm in thermal infrared is relatively simple to be installed. A box made from black cardboard displaying a little hole is suitable, as Student experiments with black cubes show. The opening appears to be of a darker black than the black cardboard. Of the cavity, infrared radiation passes out of which the spectrum approximately results in the progression of Planck's radiation law for room temperature.
For applications with high precision, where the emitter's temperature shall be variable in a range from room temperature up to 1500°C resp. 1200 K, heater ovens are used which resemble the ones used 120 years ago in the Light-technical laboratory of the Physikalisch-Technische Reichsanstalt in Berlin. High care is put into the outer isolation and even heating of the inner space to obtain a thermic equilibrium on the inside. The temperature measured by the thermometer on the inner walls will then adequately correspond to the radiation temperature of the Planck radiation coming out of the opening.

Source: Federal Institute for Metrology METAS, Bern, Switzerland
More radiation laws are presented on the pages which follow. They are in parts mathematically ambitious and not required for a first understanding. You may directly proceed to the pages about solar radiation.